-
About
- Leadership & Faculty
- News & Events
-
Admissions
-
Academics
- Graduate
- Advanced Clinical Training
- Continuing Education
- Academic Departments
- Academic Offices
- Simulation Experiences
-
Student Life
- Offices
-
Research
-
- Transformative Research
- Centers & Shared Resources
-
-
Hospitals & Clinics
- Emergency Care
- Hospital Services
-
Community Outreach
- Volunteer
Development and Application of VHH-Based Therapeutic Agents
The Shoemaker lab in the Department of Infectious Disease and Global Health develops therapeutic biomolecules based on single-domain antibody (sdAb) binding agents that are engineered into multiple scaffolds. The sdAb agents derive from the VH region of heavy-chain-only Abs (VHHs) obtained from alpacas immunized with the target antigen. VHH agents are small proteins (14 kDa) that are produced economically at high levels, more stable to temperature and pH extremes than conventional antibodies, and commonly block (neutralize) the function of targets to which they bind. In recent years, we have extensively refined our original VHH discovery platform (Maass et al) such that we typically obtain very large and diverse pools of high-affinity binding agents to each biomolecule that we ‘target’ in our search for binding agents (e.g. Vance et al). From our pool of VHHs, we identify VHHs that most potently bind to the target and/or neutralize target function. Where possible we identify neutralizing VHHs that bind at different (non-overlapping) sites on the target. These VHHs are then engineered in various ways as components of novel agents with improved in vivo therapeutic efficacies, and sometimes as diagnostics. We also have ongoing or completed collaborations with over a dozen companies in the development of VHH-based agents to targets selected by the companies.
VHH-based neutralizing agents (VNAs)
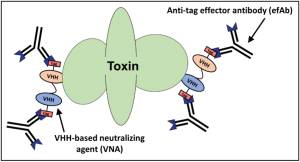
For many of our therapeutic strategies, we produce ‘multispecific’ proteins in which we genetically link two (or more) VHHs that each neutralize the therapeutic target to create VHH-based neutralizing agents (VNAs) (see Mukherjee et al). The different VHH components preferentially neutralize target functions at different sites. VNAs consisting of two different neutralizing VHHs have demonstrated dramatically improved in vivo efficacy in numerous toxin models (see Novel antitoxin agents), as well as in other infectious disease models (see Anti-infective VHH-based agents).
In many cases, we link neutralizing VHHs to more than one target such that a single VNA can target more than one pathogenic biomolecules, such as two different toxins produced by the same pathogen (see Tremblay et al and Yang et al), for improved therapeutic benefit. We have successfully targeted and neutralized three different botulism serotypes with a single hexaspecific VNA.
VNAs have proven to be ideal for gene therapy approaches. For example, in collaboration with the Curiel lab at WUSTL, we have produced adenovirus vectors that lead to the expression of therapeutic serum VNA levels in treated animals and protection from exposures to toxins (see Mukherjee et al) or toxin-mediated disease pathogens (see Sheoran et al and Moayeri et al). Working with the Lodish lab at MIT, we developed engineered red blood cells that expressed functional antitoxins on their surface that can provide long-term toxin protection following transfusion (Huang et al). In a recent collaboration with Curevac, we demonstrated antitoxin efficacy in mice treated with packaged mRNA gene therapy agents (Thran et al).
Effector Ab (efAb) for enhanced in vivo efficacies
Our VNA technology offers the novel capability for therapeutic efficacy to be substantially enhanced by the co-administration (or co-expression in gene therapy) of an effector antibody (efAb) to provide the effector functions of conventional antibody Fc domains. The efAb is an antibody recognizing a small peptide ‘tag’ motif that is engineered at one or more sites within each VNA. As shown in the figure (above), by including two tags on a bispecific VNA, the target protein becomes ‘decorated’ by up to four efAb Fc domains, and this valency promotes rapid serum clearance of the target protein (see Sepulveda et al). Employing co-administered efAb has been shown to improve antitoxin efficacy in several animal models of intoxication (see Mukherjee et al, Tremblay et al and Vance et al). The efAb itself can be engineered to possess the Fc isotype(s) required to most effectively achieve desired therapeutic benefits. In addition, the same efAb can be engineered for the animal model needed for efficacy testing, or it can be ‘humanized’ for applications in people. Importantly, the same efAb can be used for all VNA therapies since the same tag motif is incorporated into all VNAs.
Other applications of VHHs
VHHs, as high affinity and highly specific binding agents, have numerous additional potential applications beyond their use in VNAs. One application that has shown great promise is to employ VHHs that neutralize the protease of Botulinum neurotoxins (BoNTs) in the development of Botulism antidotes, agents that can ‘reverse’ the paralysis of botulism patients. Secondly, we have ongoing collaborations to develop VHHs for diagnostic applications (e.g. Dinh et al, Aberjon et al). Finally, VHHs are proving to be valuable as research tools. For example, because VHHs tend to recognize protein conformations, they can be used to probe for different conformations exhibited by the same target protein.
Selected related publications
- Tremblay JM, Vazquez-Cintron E, Lam KH, Mukherjee J, Bedenice D, Ondeck CA, Conroy MT, Bodt SML, Winner BM, Webb RP, Ichtchenko K, Jin R, McNutt PM, Shoemaker CB. 2020. Camelid VHH Antibodies that Neutralize Botulinum Neurotoxin Serotype E Intoxication or Protease Function. Toxins (Basel). 2020 Sep 24;12(10):611. doi: 10.3390/toxins12100611.
- Lam KH, Perry K, Shoemaker CB, Jin R. 2020. Two VHH antibodies neutralize Botulinum neurotoxin E1 by blocking is membrane translocation in host cells. Toxins (Basel). 2020 Sep 27;12(10):616. doi: 10.3390/toxins12100616.
- Lam KH, Tremblay JM, Vazquez-Cintron E, Perry K, Ondeck C, Webb RP, McNutt PM, Shoemaker CB, Jin R. 2020. Structural Insights into Rational Design of Single-Domain Antibody-Based Antitoxins against Botulinum Neurotoxins. Cell Rep. 30:2526-2539.
- Abeijon C, Dilo J, Tremblay JM, Viana AG, Bueno LL, Carvalho SFG, Fujiwara RT, Shoemaker CB, Campos-Neto A. 2018. Use of VHH antibodies for development of antigen detection test for visceral leishmaniasis. Parasite Immunol. e12584.
- Vance DJ, Tremblay JM, Rong Y, Angalakurthi SK, Volkin DB, Middaugh CR, Weis DD, Shoemaker CB, Mantis NJ. 2017. High-Resolution Epitope Positioning of a Large Collection of Neutralizing and Non-Neutralizing Single Domain Antibodies on Ricin Toxin's Enzymatic and Binding Subunits. Clin Vaccine Immunol. Oct 11. pii: CVI.00236-17.
- Vazquez-Cintron EJ, Beske PH, Tenezaca L, Tran BQ, Oyler JM, Glotfelty EJ, Angeles CA, Syngkon A, Mukherjee J, Kalb SR, Band PA, McNutt PM, Shoemaker CB, Ichtchenko K. 2017. Engineering Botulinum Neurotoxin C1 as a Molecular Vehicle for Intra-Neuronal Drug Delivery. Sci Rep. Feb 21;7:42923.
- Yao G, Lam KH, Weisemann J, Peng L, Krez N, Perry K, Shoemaker CB, Dong M, Rummel A, Jin R. 2017. A camelid single-domain antibody neutralizes botulinum neurotoxin A by blocking host receptor binding. Sci Rep. 7(1):7438.
- Thran M, Mukherjee J, Pönisch M, Fiedler K, Thess A, Mui BL, Hope MJ, Tam YK, Horscroft N, Heidenreich R, Fotin-Mleczek M, Shoemaker CB, Schlake T. 2017. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol Med. e201707678.
- Barta ML, Shearer JP, Arizmendi O, Tremblay JM, Mehzabeen N, Zheng Q, Battaile KP, Lovell S, Tzipori S, Picking WD, Shoemaker CB, Picking WL. 2017. Single-domain antibodies pinpoint potential targets within shigella invasion plasmid antigen D of the needle tip complex for inhibition of type III secretion. J Biol Chem. M117.802231. PMCID: PMC5633129
- Huang NJ, Pishesha N, Mukherjee J, Zhang S, Dhesycka R, Sudaryo V, Dong M, Shoemaker CB, Lodish H. 2017. Genetically engineered red cells expressing single chain camelid antibodies confer long-term protection against botulinum neurotoxin. Nat Commun. 4:423.
- Vance DJ, Tremblay JM, Rong Y, Angalakurthi SK, Volkin DB, Middaugh CR, Weis DD, Shoemaker CB, Mantis NJ. 2017. High-Resolution Epitope Positioning of a Large Collection of Neutralizing and Non-Neutralizing Single Domain Antibodies on Ricin Toxin's Enzymatic and Binding Subunits. Clin Vaccine Immunol. Oct 11. pii: CVI.00236-17.
- Dinh TL, Ngan KC, Shoemaker CB, Walt DR. 2017. Rapid and Ultrasensitive Detection of Botulinum Neurotoxin Serotype A1 in Human Serum and Urine using Single Molecule Array Method. Forensic Toxicology. Forensic Toxicol. 35:179–184.
- Vrentas CE, Moayeri M, Keefer AB, Greaney AJ, Tremblay J, O'Mard D, Leppla SH, Shoemaker CB. 2016. A diverse set of single-domain antibodies (VHHs) against the anthrax toxin lethal and edema factors provides a basis for construction of a bispecific agent that protects against anthrax infection. J Biol Chem. 2016 Aug 18. pii: jbc.M116.749184. [Epub ahead of print]
- Schmidt DJ, Beamer G, Tremblay JM, Steele JA, Kim HB, Wang Y, Debatis M, Sun X, Kashentseva EA, Dmitriev IP, Curiel DT, Shoemaker CB, Tzipori S. 2016. A Tetraspecific VHH-based Neutralizing Antibody Modifies Disease Outcome in Three Animal Models of Clostridium difficile Clin Vaccine Immunol. Jul 13. pii: CVI.00730-15.
- Moayeri M, Tremblay JM, Debatis M, Dmitriev IP, Kashentseva EA, Yeh AJ, Cheung GY, Curiel DT, Leppla S, Shoemaker CB. 2016. Adenoviral expression of a bispecific VHH-based neutralizing agent targeting protective antigen provides prophylactic protection from anthrax in mice. Clin Vaccine Immunol. CVI.00611-15.
- Herrera C, Tremblay JM, Shoemaker CB, Mantis NJ. 2015. Mechanisms of Ricin Toxin Neutralization Revealed through Engineered Homodimeric and Heterodimeric Camelid Antibodies. J Biol Chem. 2015 Sep 22. pii: jbc.M115.658070. [Epub ahead of print]
- Moayeri M, Leysath CE, Tremblay JM, Vrentas C, Crown D, Leppla SH, Shoemaker CB. 2015. A Heterodimer of a VHH Antibody that Inhibits Anthrax Toxin Cell Binding Linked to a VHH that Blocks Oligomer Formation is Highly Protective in an Anthrax Spore Challenge Model. J Biol Chem. 290:6584-95. PMCID:PMC4358291
- Yang Z, Schmidt D, Liu W, Li S, Shi L, Sheng J, Chen K, Yu H, Tremblay JM, Chen X, Piepenbrink KH, Sundberg EJ, Kelly CP, Bai G, Shoemaker CB, Feng H. 2014. “A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficileinfection in mice”. J. Inf. Dis. Sep 15;210(6):964-72.
- Rudolph MJ, Vance DJ, Cheung J, Franklin MC, Burshteyn F, Cassidy MS, Gary EN, Herrera C, Shoemaker CB, Mantis N. 2014. Crystal Structures of Ricin Toxin’s Enzymatic Subunit (RTA) in Complex with Neutralizing and Non-neutralizing Single Chain Antibodies. J Mol Biol. 426(17):3057-68. PMCID:PMC4128236.
- Herrera C, Vance DJ, Eisle L, Shoemaker CB, Mantis N. 2014. Differential Neutralizing Activities of a Single Domain Camelid Antibody (VHH) Specific for Ricin Toxin’s Binding Subunit (RTB). PLoS ONE. Jun 11;9(6):e99788. PMCID:PMC4053406.
- Kaliberov SA, Kaliberova LN, Buggio M, Tremblay JM, Shoemaker CB, Curiel DT. 2014. Adenoviral targeting using genetically incorporated camelid single variable domains. Lab. Invest. 94(8):893-905. PMCID:PMC4157633.
- Mukherjee J, Dmitriev i, Debatis M, Tremblay JM, Beamer G, Kashentseva EA, Curiel DT, Shoemaker CB. 2014. Prolonged prophylactic protection from botulism with a single adenovirus treatment promoting serum expression of a VHH-based antitoxin protein. PLoS ONE. Aug 29;9(8):e106422. PMCID:PMC4149568.
- Barrera D, Rosenberg J, Chiu J, Chang YN, Debatis M, Ngoi SM, Chang J, Shoemaker CB, Oyler G, Mayfield S. 2014. Algal chloroplast produced camelid VHH anti-toxins are capable of neutralizing botulinum neurotoxin. Plant Biotech. J. 13:117-24. PMCID:PMC4620920
- Sheoran AS, Dmitriev IP, Kashentseva EA, Cohen O, Mukherjee J, Debatis M, Shearer J, Tremblay JM, Beamer G, Curiel DT, Shoemaker CB, Tzipori S.. 2014. Adenovirus vector expressing Stx1/2-neutralizing agent protects piglets infected with E. coli O157:H7 against fatal systemic intoxication. Infect Immun. 83:286-91. PMCID: PMC4288880
- Vance DJ, Tremblay JM, Mantis NJ, Shoemaker CB. 2013. Stepwise engineering of heterodimeric single domain camelid VHH antibodies that passively protect mice from ricin toxin. J. Biol. Chem. Dec 20;288(51):36538-47. PMCID:PMC3868766.
- Tremblay JM, Mukherjee J, Leysath CE, Debatis M, Ofori K, Baldwin K, Boucher C, Peters R, Beamer G, Sheoran A, Bedenice D, Tzipori S, Shoemaker CB. 2013. A single VHH-based toxin neutralizing agent and an effector antibody protects mice against challenge with Shiga toxins 1 and 2. Infect Immun. Dec;81(12):4592-603. doi: 10.1128/IAI.01033-13. Epub 2013 Sep 30. PMCID: PMC3837998
- Mukherjee J, Tremblay JM, Leysath CE, Ofori K, Baldwin K, Feng X, Bedenice D, Webb RP, Wright PM, Smith LA, Tzipori S, Shoemaker CB. 2012. A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS ONE. 7(1):e29941. PMCID: PMC3253120
- Kuo CL, Oyler GA, Shoemaker CB. 2011. Accelerated neuronal cell recovery from Botulinum neurotoxin intoxication by targeted ubiquitination. PLoS ONE. 6(5):e20352. PMCID: PMC3101245
- Tremblay JM, Kuo CL, Abeijon C, Sepulveda J, Oyler G, Hu X, Jin MM, Shoemaker CB. 2010. Camelid single domain antibodies (VHHs) as neuronal cell intrabody binding agents and inhibitors of Clostridium botulinumneurotoxin (BoNT) proteases. Toxicon. 56:990-8. PMCID: PMC2946066
Maass, DM, Sepulveda, J, Pernthaner, A, Shoemaker, CB. 2007. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs). J Immunol Methods. 324:13-25. PMCID: PMC2014515