Highly Specialized Support Team
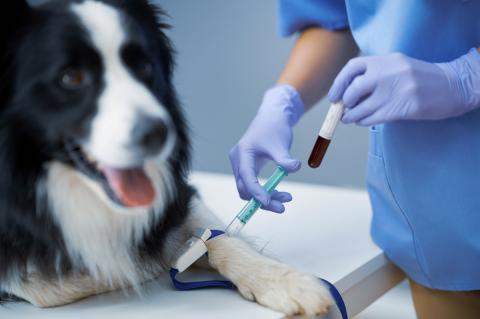
Your pet will receive care from a highly specialized support team of veterinarians and veterinary nurses who monitor progress in the study very closely.
Clinical trials at Cummings School of Veterinary Medicine at Tufts University can offer your animal hope when there may not be other options, and some can even lead to the advancement in both human and veterinary medicine. Established in 2017, the Clinical Trials Office at Cummings School of Veterinary Medicine oversees a variety of trials involving a wide spectrum of diseases including cancer, kidney disease, arthritis, and neurologic conditions, among others.
Each year at Cummings Veterinary Medical Center, over 500 animals participate in clinical trials, with 30 studies typically enrolling patients at any given time. Prepare for your pet’s visit by learning more about what to expect.
Do you have a patient whom you feel is eligible for a clinical trial? Learn more about referrals and the benefits of clinical trials at Cummings School of Veterinary Medicine.
The Clinical Trials Office currently has six full-time staff members including Director Dr. Cheryl London. Meet the dedicated staff.
Search or browse current clinical trials by species and area of study.
Clinical trials typically involve evaluation of a new treatment, device, diagnostic test, or procedure. The ultimate goal of this work is to improve the health and well-being of veterinary patients through such things as better care or earlier diagnosis. In some cases, a study may be very short (i.e., one day) and in others, several visits may be required. Before enrolling in a clinical trial, the study staff and attending veterinarians at Cummings Veterinary Medical Center will evaluate your pet to determine whether s/he is eligible to enroll, and they will also provide you with every available option for diagnosis and treatment outside of the study.
Your pet will receive care from a highly specialized support team of veterinarians and veterinary nurses who monitor progress in the study very closely.
Typically some, if not all, of the costs of participating in the clinical study, including medications, blood tests, treatments and follow-up, may be covered. Additionally, many studies provide a financial credit at for future treatments once the trial is complete.
In some cases, a clinical study in which your pet may participate will not only help advance the care of veterinary patients, but will also provide important new information that can improve the care of human patients affected by the same disease.
Each trial is led by a lead principal investigator (PI), with other veterinarians and staff providing expertise and support. All clinical studies are rigorously evaluated by the Clinical Studies Review Committee (CSRC) and the Institutional Animal Care and Use Committee (IACUC) prior to approval.
The CSRC is a group of doctors and experts that reviews the detailed plan of a clinical trial for scientific quality and correct study design, serves to ensure informed consent and protect animals from conflict of interest issues. The IACUC reviews research protocols and conducts evaluations of the animal care and use, which includes the results of inspections of facilities.
If you are interested in enrolling your pet into a study, you will receive a consent form that provides details about the study, your responsibilities/commitments associated with the study, potential risks and benefits, and what financial considerations are provided following enrollment. You will be given opportunities to discuss the study with your family/friends, regular veterinarian, and study personnel. Should you then decide to enter your pet into the study, you will be asked to sign the consent form (this process is termed Informed Consent).
Most studies require several visits to Foster Hospital for Small Animals, although some studies are relatively simple and only one visit is required. In certain instances, your pet may need to stay overnight for treatment or monitoring associated with the study. At all times during any clinical study, the health and well being of your pet are monitored very closely by the study team.
The success of any clinical trial depends entirely on recruitment and enrollment of an appropriate population of patients. Foster Hospital for Small Animals has a broad array of trials currently underway ranging from cancer to arthritis.
Please explore a comprehensive and updated list of our current clinical trials. The study requirements are also provided so you can often quickly determine if your pet may be eligible for a study.
If you believe that your pet may be eligible for a study, please complete this questionnaire. Once submitted, a member of the Clinical Trials Office will contact you within one to two business days. Your family veterinarian may also contact us directly for more information or complete this referring veterinarian questionnaire.
We look forward to working with you on the most state-of-the-art and innovative therapies. If you have any questions about a particular trial or eligibility, please contact the Clinical Trials Office at clinicaltrials@tufts.edu.




Clinical Trials Office
55 Willard St.
N. Grafton, MA 01536
Phone: 508-887-4441
Fax: 508-887-4880
Email: clinicaltrials@tufts.edu