-
About
- Leadership & Faculty
- News & Events
-
Academics
- Graduate
- Advanced Clinical Training
- Continuing Education
- Academic Departments
- Academic Offices
- Simulation Experiences
-
Student Life
- Offices
-
Research
-
Hospitals & Clinics
- Emergency Care
- Hospital Services
-
Community Outreach
- Volunteer
A Poster Child for Progress
Cummings School of Veterinary Medicine and Tufts teamC researching new technologies to advance treatment of heart defect present at birth
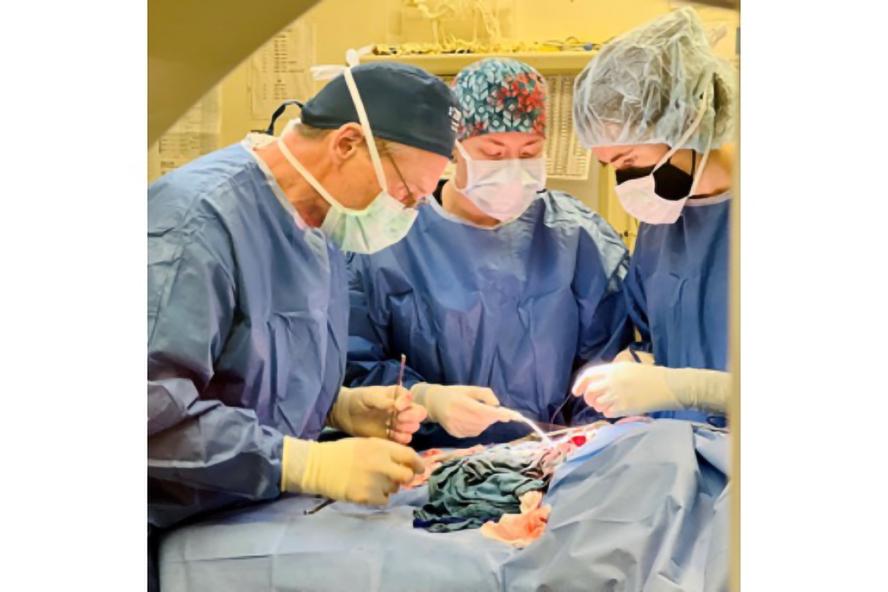
Tetralogy of Fallot, a life-threatening congenital heart disease affecting children, currently requires multiple major surgeries beginning at infancy. More than 1,500 babies are estimated to be diagnosed with it each year, which sees four defects that combine to cause the inability to oxygenate blood properly and cannot be survived without intervention.
As it stands, treatment requires an initial surgery to place a Dacron or Teflon patch into the stenosed, or restricted, vessel to allow blood to be appropriately pushed through the lungs. But, this form of treatment has limits because the Teflon does not stretch, dissipate, or grow with the child, so a second major surgery is required.
A team of Tufts researchers are aiming to prevent additional surgeries for affected children, however. Dr. Raymond Kudej, small animal surgeon at the Henry and Lois Foster Hospital for Small Animals at Cummings School of Veterinary Medicine, was approached by Drs. Lauren Black and David Kaplan of Tufts University’s Department of Biomedical Engineering, to determine the feasibility of a new, more adaptable patch and to create a successful model and implantation technique to test the implant.
In the first iteration of their research, Black and Kaplan created a new patch out of silk biomaterial that can change properties and dissolve at different rates of time. They also wove stem cells into the scaffolding of the patch. “The whole concept involved creating a patch that could be used for smaller patients that will grow and integrate with the heart, so they wouldn’t need the surgery again,” says Kudej. “So that's where the silk based material comes in. It's used for a lot of different things but they can kind of manipulate how rapidly the structure of it dissolves. And then the stem cells would reproduce and start to regenerate that heart muscle as the scaffolding disappears, so it won't restrict that area of the heart from growing with the rest of the patient.”
Kudej, a fellow of the American Heart Association who has performed the surgeries at Cummings School, has extensive experience with pig models and worked with numerous models of cardiac disease in humans during his postdoctoral studies at Harvard Medical School. Because a pig heart is very similar to a human heart, the research model best mimics how it might work in a child or a growing patient.
During the study, Kudej either implanted a Dacron patch like one currently used in humans, the silk-based patch without any stem cells, or the silk-based patch with stem cells. The first generation of the study saw promising results. “We were pretty successful from the start; we really didn't have any failures of the patch,” explains Kudej. “The inflammation is what causes scar tissue, so that's where the problems were with the first round of this research.”
Now, the research team has applied for a second generation of the study, where they’ll focus on harvesting from within the stem cells, the parts that are responsible for the tissue development and regeneration. The objective will be to decrease the inflammatory response to the patch, and in turn, see it integrate and grow with the patient.
“This is a new technology and the model is good,” says Kudej. “It's heartbreaking for a child to go through these major thoracic surgeries. Any time you go in a second time for surgery, everything's more difficult for the surgeon and more difficult for the patient, too. So, the idea of this study is to advance medicine, and this is the safest way.”